Bio2Bedside
Neobe Therapeutics: Genetically-Engineered, ECM-Degrading Bacteria for Solid Tumors
Neobe Therapeutics
Neobe is a UK-based biotech engineering tumor-targeting bacterial therapies to dismantle fibrotic barriers in solid tumors.
Ask us anything about Neobe
Neobe Tx: Episode Overview
Check out our episode overview to see how Neobe is harnessing tumor-homing bacteria to address the thick ECM of solid tumors.
Neobe’s Story: Featuring CEO & Co-Founder Pedro Correa de Sampaio, PhD

B2B Brief: Neobe Therapeutics
Overview
Neobe Therapeutics is a UK-based biotech developing engineered bacterial therapies designed to break down fibrotic barriers in solid tumors. By targeting the tumor microenvironment – not the tumor cells directly – Neobe enables more effective penetration of immune cells, biologic therapies, and even things like chemotherapy, potentially transforming outcomes in traditionally refractory cancers.
Leadership
Founded by cancer biologist Pedro Correa de Sampaio, PhD (PhD from Cambridge, post-doc MD Anderson) and bacterial engineer Annelise Soulier, Neobe was launched through Deep Science Ventures and brings together multidisciplinary expertise in tumor biology, microbial engineering, and synthetic biology.
Scientific Overview
Neobe’s platform uses commensal-derived, live bacteria engineered with biosensors that detect tumor-specific environmental cues (such as hypoxia, low pH, and nutrient profiles). Once inside the tumor, these bacteria locally secrete ECM-degrading enzymes (e.g., hyaluronidase), “softening” the tumor and improving perfusion, immune infiltration, and ultimately therapeutic efficacy.
Clinical Plans & Potential Impact
Preclinical studies in triple-negative breast cancer and pancreatic cancer models show complete responses in tumors previously resistant to checkpoint inhibitors. The company aims to enter clinical trials in 2027, positioning its platform as a tumor-priming modality for use alongside immune checkpoint inhibitors, CAR-T cells, ADCs, and other therapies.

Due Diligence
The Good
This is new:
Neobe is tackling a long-overlooked problem in oncology: immune and drug exclusion due to dense, fibrotic tumor stroma. Their platform is differentiated by its non-immunogenic, extracellular, and programmable design, offering broad compatibility with existing cancer therapeutics.
This is different:
Unlike other microbial immunotherapy companies focused on immune activation, Neobe is remodeling the tumor microenvironment to remove physical barriers and boost response rates across multiple drug classes
The Risks
Biological Uncertainty
Neobe’s microbes rely on sensing specific intratumoral cues – like hypoxia, acidity, and metabolite shifts – to activate ECM-degrading enzyme release. While these conditions are commonly associated with dense fibrotic tumors, they aren’t universally present.
That raises a key question:
Could some tumors have a thick ECM but lack the microenvironmental “activation signals” required to trigger Neobe’s payload? If so, efficacy could vary tumor-to-tumor, potentially limiting the platform’s reliability in certain settings.
Regulatory Hurdles:
As a live bacterial gene therapy, Neobe faces regulatory and safety complexities. Although the platform uses commensal strains and includes safety controls (e.g., non-pathogenic chassis, plasmid stabilization, antibiotic sensitivity), clinical validation of biodistribution, persistence, and redosing feasibility remains a key inflection point.
Unorthodox Manufacturing:
Manufacturing coordination between microbial production and sterile fill-finish also presents operational hurdles – the “sterile fill-finish” folks tend to not like bacteria…you see the problem.
The Competition
Others exist:
Several companies like Salspera, Actym, and T3 Pharma are developing other bacteria-based cancer therapeutics. Most use attenuated pathogens to deliver immune-stimulating payloads into tumors.
But Neobe is different:
In contrast, Neobe uses commensal-derived bacteria to remodel the extracellular matrix, improving drug and immune cell infiltration. Rather than activating the immune system directly, Neobe acts as a tumor-priming platform, enabling other therapies to work more effectively by breaking down physical barriers within the tumor microenvironment.
Other show highlights
The Next Big Thing: Bacteria-Based Therapy

Concise scientific overview of Neobe's technology

Won't the bacteria cause an infection?

If antibodies are too big, won't bacteria be too big?

Recent Features

Neobe Therapeutics
Founded:
2021
Location:
London, England
Stage:
IND-Enabling
Status:
Private
Funding to date:
$2.3M
Share profile:
Key Leadership
Notable Investors



Meet the Hosts
Recent Features

Published: 09 May 2024
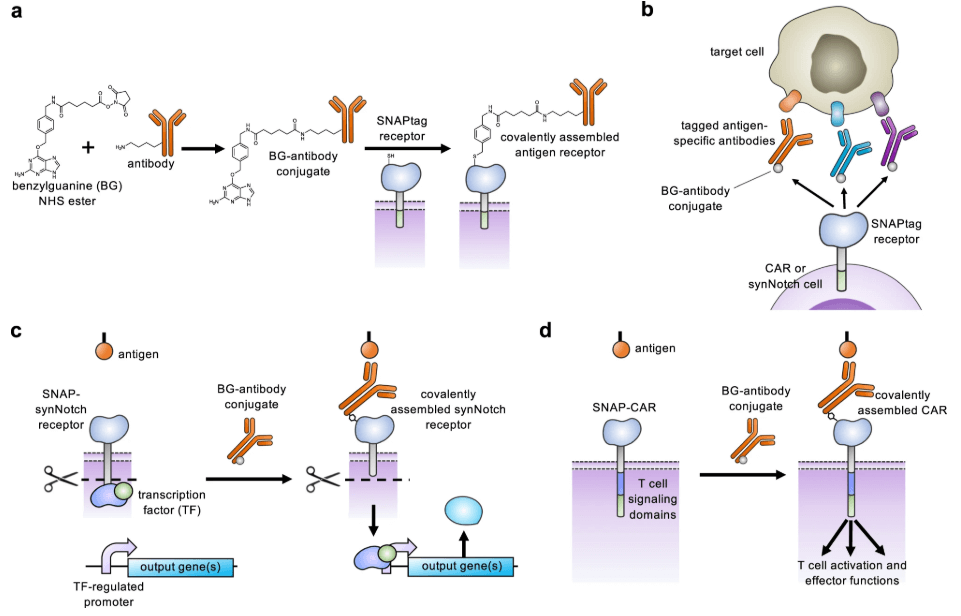
Post-translational covalent assembly of CAR and synNotch receptors for programmable antigen targeting
*Featured on cover! Volume 8 Issue 12

Published: 19 March 2025
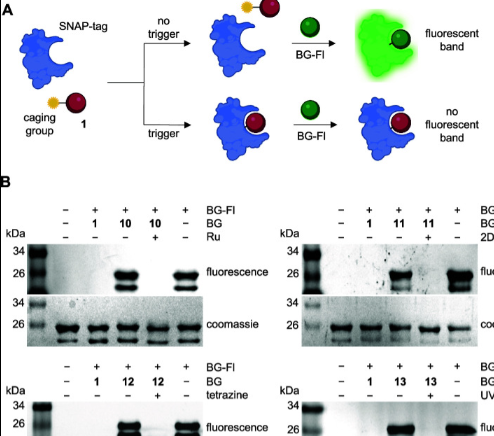
Conditional Control of Benzylguanine Reaction with the Self-Labeling SNAP-tag Protein
*Featured on cover! Volume 8 Issue 12
Featured Media
No recent media.
Recent News
No recent news.
Job Postings
No job postings at this time.