MaxCyte
MaxCyte is a U.S.-based biotechnology company that develops and commercializes cell-engineering technologies used in both research and therapeutic manufacturing.
MaxCyte: Episode Overview
We discuss why viral and non-viral delivery will coexist – ex vivo for complex, multi-edit therapies and LNPs for improved targeting, persistence, and redosing in vivo.
Episode Highlights
Andrew Mancini + MaxCyte: An Introduction

The "electrical dance" of modern electroporation.

How do LNPs remain competitive into the future?

Achieving complex ex vivo cell engineering.

The Limitations of Viral-Based Cell Engineering


B2B Brief: NAYA Therapeutics
Overview
Naya Therapeutics focuses on patients with hepatocellular carcinoma (HCC) who fail to respond to checkpoint inhibitors or chemotherapy. The company is advancing a dual-modality strategy: NK cell engagers to restore innate immune function and alpha radiopharmaceuticals to eradicate residual disease.
This approach directly addresses the limitations of current HCC therapies, where response rates remain below 30%.
Leadership
Led by Daniel Teper, PharmD, MBA (Chairman & CEO), Naya’s leadership team brings significant experience in oncology innovation, financing, and commercialization. Executives include Michael G. King (EVP), Raquel Cabo (COO), Lyn Falconio (CCO), Dan Chiche, MD (CMO), Vidisha Mohad, PhD (Head of Product Development), and Ravi Kiron, PhD, MBA (CBO). The board and advisors feature veterans from Sanofi, Amgen, Pfizer, and venture firms .
Naya’s clinical program is guided by Prof. Yaron Ilan (featured in this episode), a hepatology leader and former President of the Israeli Liver Association, with decades of experience in liver cancer and chronic liver disease.
Scientific Overview
Naya’s NK cell engager platform leverages NKp46 activation to restore functionality in exhausted NK cells and redirect them against GPC3-expressing tumors, the dominant biomarker in HCC.
In parallel, its radiopharmaceutical platform employs antibody fragments conjugated with astatine-211 (At-211), an alpha-emitter with a short half-life, simple decay chain, and covalent binding chemistry, enabling high tumor penetration and outpatient administration.
Clinical Plans & Potential Impact
The lead NK cell engager will enter Phase 1 clinical testing in checkpoint inhibitor non-responders in HCC.
The radiopharmaceutical program aims to establish proof-of-concept in minimal residual disease settings, with potential expansion to other solid tumors.
Together, the two modalities are designed to raise HCC response rates well above current standards, with global relevance given rising HCC incidence.

Due Diligence
The Good
This is different:
Unlike T-cell therapies, which risk collateral damage in cirrhotic livers, NK biology is naturally aligned with liver immunity. The liver harbors a high proportion of NK and NKT cells, making them the frontline defense against malignant transformation.
Naya’s unique patient fit:
Most HCC patients present with advanced disease on a background of cirrhosis or failing livers, where treatment tolerance is low and systemic toxicity is often lethal. Naya’s therapeutics – precise, NK-driven cytotoxicity and short-lived, clean-decay alpha therapy – are designed to minimize collateral liver injury while targeting residual disease.
Naya’s strategic advantage:
By tailoring therapies to the unique physiology and vulnerability of late-stage liver patients, Naya occupies a niche where checkpoint inhibitors and T-cell therapies often fail due to poor response rates or unacceptable toxicity.
The Risks
Therapeutic:
No clinical proof yet for NAYA’s strategy of NK cell engagers and ²¹¹At radiopharmaceuticals in HCC; efficacy and safety remain to be demonstrated.
Logistical:
Manufacturing bispecifics is complex; ²¹¹At’s short half-life complicates supply/distribution; recruiting post–checkpoint inhibitor HCC patients is challenging.
Regulatory:
First-in-class modalities face uncertain FDA/EMA pathways; heightened CMC/dosimetry scrutiny; reimbursement risks if costs outweigh incremental benefit over existing standards.
The Competition
NK cell engagers:
Innate Pharma, Dragonfly, and Affimed are developing NK engager platforms, with most programs focused on hematologic malignancies or using CD16 as the engager. Naya differentiates by targeting NKp46, a receptor with stronger and more durable activation, particularly relevant in solid tumors such as HCC.
Targeted alpha therapies (TATs):
Bayer and RayzeBio/BMS are advancing GPC3–Actinium-225 radiopharmaceuticals for HCC, while Novartis and AstraZeneca have made large bets in PSMA and other alpha radioligands. Naya’s advantage is the use of astatine-211, which has a short half-life, a clean single decay path, and outpatient feasibility, addressing many limitations of actinium and lutetium approaches.
But this company is different:
By focusing squarely on HCC biology and aligning modality to setting — NKp46 engagers for refractory tumors and At-211 radiopharma for micro-metastatic disease — Naya avoids the “one-size-fits-all” strategy and positions itself in niches where existing competitors are less well-suited.

MaxCyte
Founded:
1998
Location:

Rockville, MD, USA
Clinical Stage:
Phase I Prep
Status:
post-IPO
Funding to date:
~$15M
Share profile:
Key Speakers
Meet the Hosts
Recent Features

Published: 09 May 2024
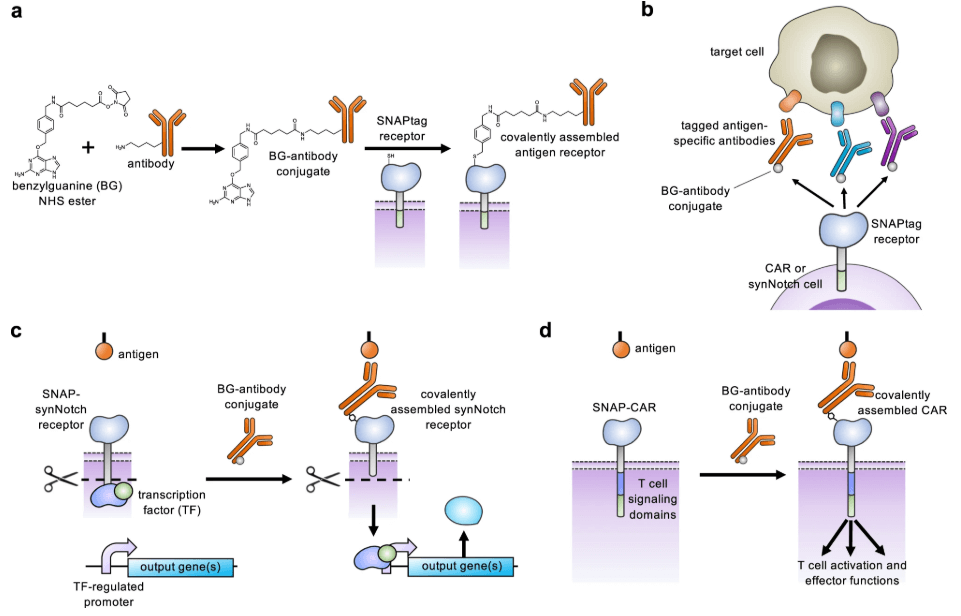
Post-translational covalent assembly of CAR and synNotch receptors for programmable antigen targeting
*Featured on cover! Volume 8 Issue 12

Published: 19 March 2025
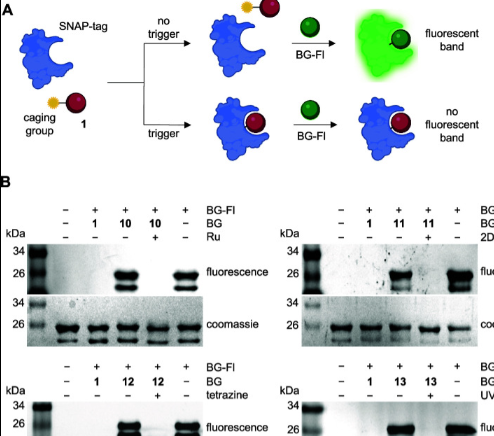
Conditional Control of Benzylguanine Reaction with the Self-Labeling SNAP-tag Protein
*Featured on cover! Volume 8 Issue 12
Featured Media
No recent media.
Recent News
No recent news.
Job Postings
No job postings at this time.