Bio2Bedside
Featured Biotech
KiraGen Bio: AI-Guided Multi-Genic CAR-T Cells for Solid Tumors
KiraGen Bio
KiraGen Bio is developing multi-plex gene-edited CAR-T cell therapies for solid tumors.
Key Highlights

Company Snapshot
Overview
The Challenge: Cell therapies have become too expensive, toxic, and difficult to develop. Patients deserve access, but too many barriers exist.
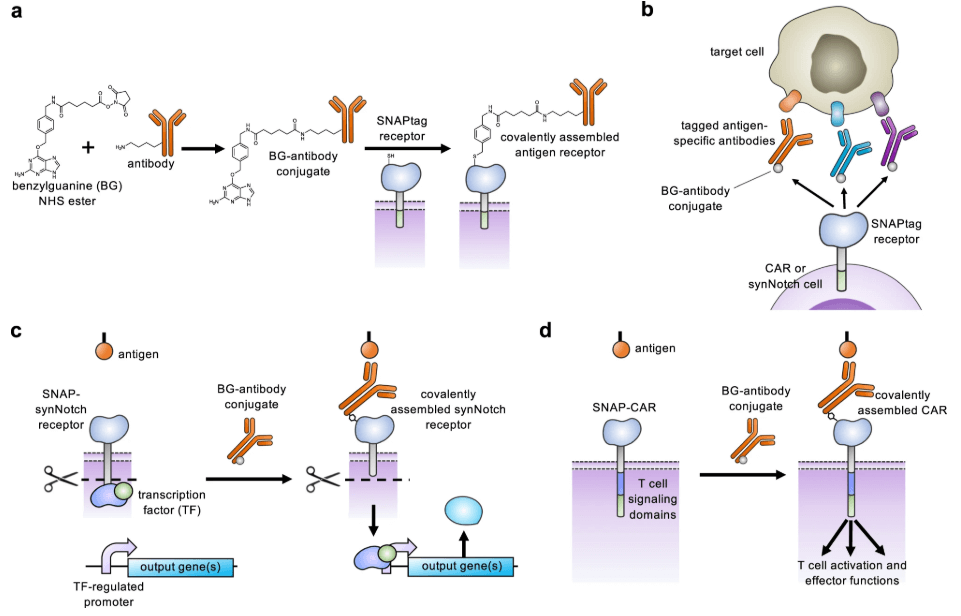
SNAP’s Approach: SNAP-CAR is a universal CAR receptor that enables already existing antibodies to be used as targeting molecules for cell therapies, offering a path to modular, programmable immune cell products.
Real-World Impact: The ability to repurpose already existing antibodies as targeting modules could accelerate clinical translation and expand access to cell-based therapies to a broad array of patients.
The Team: SNAP’s team has deep experience in advancing oncology-focused cell therapy programs from early development through clinical translation and regulatory engagement.
Clinical Development
Lead Program: SNAP-NK: Allogeneic NK cells derived from donor peripheral blood and engineered to express SNAP-CAR receptor.
Current Status: With strong preclinical validation and a flexible platform design, SNAP is strategically evaluating indication selection and development partnerships to align its first-in-human programs with the greatest clinical and commercial impact.
Delivery Method: Intravenous infusion of engineered SNAP-CAR cells – similar to other CAR-based cell therapies – co-administered with modified antibodies.
Stage: Preclinical
Impact & Market Opportunity
Target Indication: Initial focus will be CD19/CD20+ B cell malignancies affecting over 75,000 patients annually in the U.S. alone.
Clinical Burden: Five-year survival rates for aggressive B cell malignancies range from 40–70%, with over 20,000 related deaths annually in the U.S.
Market Growth: A $3.5B+ global market opportunity for cell therapies across B cell malignancies, driven by increasing relapse rates and CAR-T adoption. Sources below.
Expansion Potential: The platform’s modular design enables rapid expansion into multiple indications and therapeutic areas beyond oncology, including autoimmunity and infectious diseases.
An Introduction to KiraGen Bio
Engineering Resilience into CAR-T for Solid Tumors
KiraGen Bio is developing multiplex-edited CAR-T cell therapies designed to overcome the immunosuppressive tumor microenvironment (TME) of solid tumors. The goal? To “enable cell therapies to finally have durable efficacy in solid tumors” – the holy grail of CAR-T cell therapy.
Their lead program is in glioblastoma, but their approach is broadly applicable across solid cancers (and liquid cancers broadly).
3 Major Barriers to CAR-T in Solid Tumors
Here's the plan: Get in, Find the Target, & Don't Die.
Here Aaron describes how, when it comes to cell therapy in solid tumors, there are really 3 hurdles that must be overcome. First the cells have to get into the tumor. Then, they have to find their target (the antigen). Finally, they have to be able to deal with the immunosuppressive microenvironment long enough to induce their activity.
He then goes on to explain that “a lot of progress has been made in the first 2”
i) getting the cells in, and
ii) finding a good antigen – this means the major problem is immunosuppression.
For context, he is referring to the fact that, in some indications – glioblastoma being one of them – we can kind of “skip” the first step by doing intracranial infusions which results in efficient trafficking to brain cancers. Then, there are some pretty good targets in glioblastoma like EGFRvIII and IL13Rα2.
So, in many cancers, like glioblastoma, if we want CAR-T cells to work, we really need to focus on IMMUNOSUPPRESSION.
The Adaptive Tumor Microenvironment
We NEED a Multi-Genic Approach to Overcome the TME
Okay, here’s where the conversation gets really interesting. Aaron starts to explain how KiraGen really focuses on two core insights:
1. The Tumor Microenvironment is Adaptive
The TME operates like an immune-suppressive ecosystem that can compensate and reconfigure in response to targeted pressure – “not unlike how bacteria develop resistance to antibiotics” (I am quoting Ben McLeod here). It can “sense” that you’ve taken something away and, in order to survive, will compensate by shifting towards some other immunosuppressive mechanism.
Aaron mentions how a recent study found that inhibiting a single factor like TGF-β can be quickly offset by upregulation of other suppressive mechanisms. This is why KiraGen believes a durable anti-tumor response demands a multi-layered strategy that simultaneously knocks out multiple immunosuppressive pathways.
Synthetic Biology Isn't Always The Answer
Pumping Cells Full of Stuff Might Not Be The Best Way Forward
2. Less is More: Preserve Canonical T Cell Biology
Rather than arming T cells with synthetic weapons or switch receptors that risk overstimulation and exhaustion, KiraGen takes a subtractive approach. Their platform focuses on genetic deletion of critical signaling components involved in responding to immunosuppressive signals – basically allowing CAR-T cells to “ignore” the suppressive TME rather than rewire how they interpret it.
Why do this? Well, it actually makes a lot of sense. The goal is to preserve as much natural T cell function as possible while increasing resilience in hostile environments.
For me (this is Jeff, by the way), this was the standout moment of the entire conversation – and it’s what I think truly sets KiraGen apart from the rest of the field. While many CAR-T and NK cell companies are piling on synthetic add-ons and engineered complexity, KiraGen takes a more restrained, biologically grounded approach. Instead of forcing cells to fight harder with artificial tools, they ask a simpler question: what if we just remove the brakes and let the T cells do what they’re meant to do? Well… as naturally as possible, given they’re still CAR-Ts.
Biologic WISH They Could Do This...But They Can't.
Multiple Deletions Accomplishes Something Others Can't
Here, Aaron makes a super good point.
He says that biologics WISH they could do this [block a wide array of immunosuppressive signals at once], but they can’t because to simultaneously block these pathways throughout the body, all at once would cause too many toxicities.
BUT! By simply deleting these receptors from the CAR-T cells – PD-1, TGF-ß receptor, SIRPα, etc. – you effectively CAN block all of these at once only on your CAR-T cells.
KiraGen's AI Model Is The Key Enabling Technology
So...where does the AI come in?
Aaron then goes on to say that, determining the proper combination of edits for a given disease type can be basically impossible because there are so many different permutations of a given strategy and every tumor is different.
So, to determine the optimal set of edits for a given tumor type, KiraGen integrates data from clinical studies, in-house organoid models, and public datasets into their AI-powered analysis that then maps each TME’s dominant suppressive features and recommends multiplex gene edits tailored to that environment.
This rational design approach yields highly specific, multi-edited CAR-T cells built to simultaneously overcome the suppressive nature of the TME, preserve as much natural T cell biology as possible, AND be able to adapt to the shifting nature of tumor.
KiraGen’s vision is a platform that can decode and defuse the immunosuppressive complexity of virtually any solid tumor – making CAR-T a viable option far beyond hematologic cancers.

KiraGen Bio
Founded:
2023
Location:

Allston, MA
Stage:
IND-Enabling
Status:
Private
Funding:
Raising Series A
Share profile:
Key Leadership
Relevant Literature

Published: 09 May 2024
Post-translational covalent assembly of CAR and synNotch receptors for programmable antigen targeting
*Featured on cover! Volume 8 Issue 12

Published: 19 March 2025
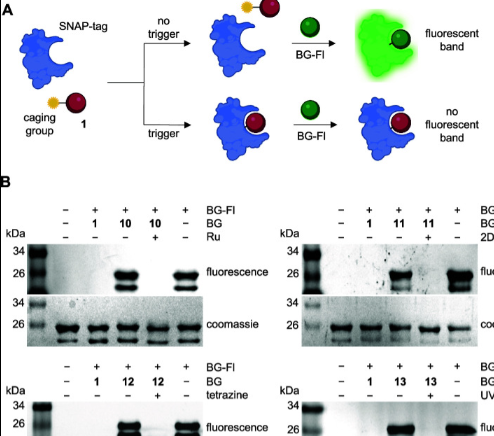
Conditional Control of Benzylguanine Reaction with the Self-Labeling SNAP-tag Protein
*Featured on cover! Volume 8 Issue 12
Featured Media
No recent media.
Recent News
No recent news.
Job Postings
No job postings at this time.